mercury free Fillings
Quality Dental Care
Dentist CHARLOTTE, NC
AnushA Chintala, DMD
PAUL PLASCYK, D.D.S
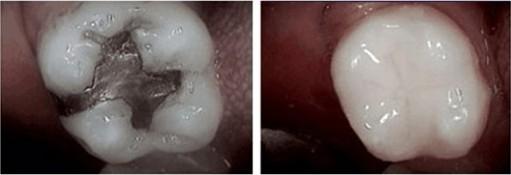
Amalgam Filling Composite and Ceramic Filling
(mercury) (mercury free)